
DG Air Freight team transporting Class 6 shipment
The National Pathology Accreditation Advisory Council (NPAAC) has set out new requirements for the packaging and transport of pathology specimens and associated materials in the fourth edition 2013. These requirements should serve as guidelines for dangerous goods specialists when transporting Class 6 infectious substances and dry ice as refrigerant for temperature sensitive samples. These are also the minimum standards set to achieve uniform practice of safe handling of pathology specimen throughout the Australia. Training, packaging, transport, and documentation are the focus of these guidelines.
Training
Training is must for all the staff responsible for packaging and transport of pathology specimens. They are required to be aptly trained and evaluated for their knowledge if they are competent enough to handle biologically hazardous shipments. Training records must be maintained by the organization and reproducible on request by the relevant authority. Trainers are required to received updated and certified training and must have current regulatory knowledge for pathology specimens.
It is mandatory for Air Operators, Freight Forwarders, Shippers, Security Screeners, Ground Handling Agents (GHAs), Australian Operators with Overseas Based Employees, and Foreign Operators with Employees Based in Australia to undertake accrediting training before performing transport and packing tasks for pathology specimens shipments.
For air freight of the infectious substances or dry ice, the dangerous goods transporters must ensure that each of their (Group F) employees involved in packaging, marking, labeling and documentation and their immediate supervisors are trained and certified before handling Class 6 dangerous goods, including pathology specimens. After every two years, they would also require recertification. Their training records must be maintained by their employers and available to be presented when requested so by the Civil Aviation Safety Authority (CASA) of Australia.
Packaging
All shipments including pathology specimens must be triple packed when transporting by air or surface. Triple packaging consists of three packing layers- Primary receptacle, secondary packaging and outer packaging. And what are the requirements for each packaging layer, is demonstrated from the image below.
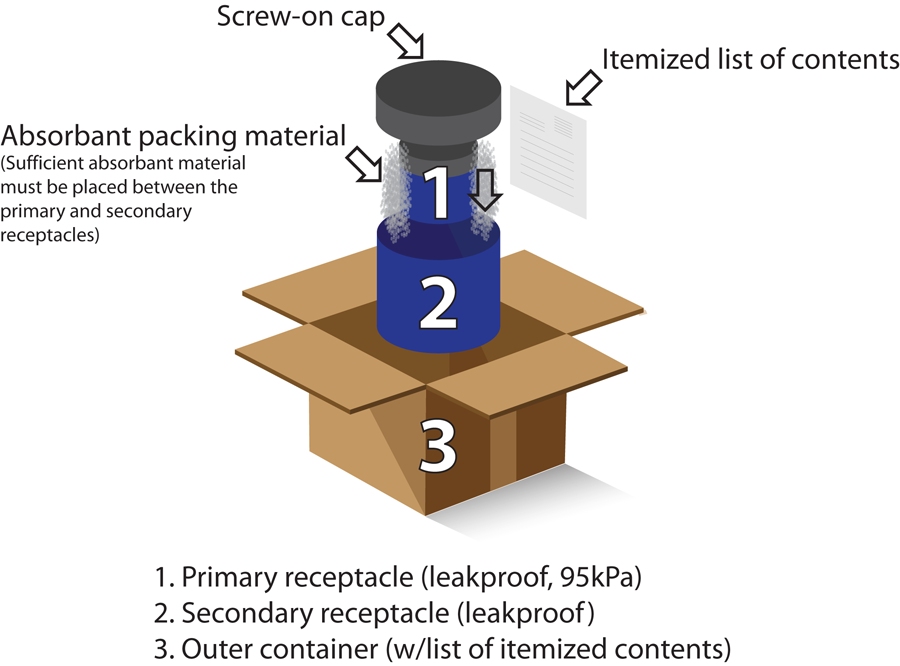
For air transport of Category A and Category B infectious substances, it must be ensured that all specimens are triple packed and the outer packaging must bear the UN packaging specification marking and test certification number respectively. For triple packaging, the primary receptacle and secondary packaging must be watertight and sufficient absorbent material must be placed between the primary receptacle and secondary packaging. However, if multiple fragile primary receptacles are placed in a single secondary packaging, they must be either individually wrapped or separated to prevent any possible contact between them during transportation. The outer packaging must be rigid and of adequate strength for its capacity, weight and intended use. The minimum size of the smallest dimension of the package must be 100mm (4 inches). The primary receptacle or secondary packaging must be capable of surviving, without leakage, against an internal pressure producing a pressure differential of not less than 95 kPa (0.95 bar, 13.8 lb/in2) and temperature in the range of –40°C to +55°C (–40°F to 130°F).
However, there are certain distinguished guidelines for air freight of both categories of infectious substances. For Category A infectious substances, the maximum net quantity per package permissible is 50 mL or 50g in passenger aircraft and 4 L or 4 kg in cargo aircraft. While for Category B infectious substances, both passenger and cargo aircrafts are allowed to carry not more than 4 L or 4 kg of pathological shipments. Furthermore, it is required that the primary receptacles of the Category B packages must not contain more than 1 L liquid and the total volume of the package must not exceed than 4 L. And for solids, the primary receptacles must be siftproof and the total package must not exceed than 4 kg.
Dry ice, since used as refrigerant for thermal approved packaging of infectious substances, packages must also comply with air transport requirements. The maximum net quantity of dry ice per package must not exceed than 2.5 kg in aircraft cabin and 200 kg in hold for passenger aircrafts and 200 kg for cargo aircrafts. When transporting dry ice by air or surface, the packaging must be designed to permit the release of carbon dioxide and to prevent the build-up pressure that may cause damage to the packaging. The dry ice is placed outside the secondary packaging and there must be sufficient interior support to keep the secondary packaging intact if the dry ice dissolves. The Dewar flask is the most commonly used container with liquid nitrogen for laboratory transport of pathology specimens.
Certainly, there are marking and labeling instructions for pathology specimens packaging. The outer packaging of pathology specimens must include:
- Name and address of sender
- Name and address of receiver
- Emergency contact details (for Category A substances, these details must be on outer packaging and for Category B substances, they may be either on outer packaging or waybill/consignment note)
- Proper shipping name
- Total volume or weight
- Hazard label
Along with, it must be noted that both Category A and Category B packages if containing 50 mL or more liquid, must display two orientation labels with each label affixed to two opposite sides and arrows must be pointing towards upside.

DG Air Freight Category B Packaging
Transport
Category A Infectious substances are prohibited for transport by Australia Post. Contrastingly, Category B biological substances are prohibited in international post. Both categories of infectious substances can be transported by air, road and rail depending upon the distance of consignees. For surface transport, the pathology specimens in racks should not be transported in public areas (lifts, corridors, etc.) unless they are protected by outer packaging. The requirement for safe transport of pathology specimens by road and rail is the same as for air- the packaged material should not have any possibility of escaping from the package under normal conditions of transport. The dry ice packages should be securely placed and restrained within the vehicle. Trucks are not recommended for transport of Category A substances. Pathology specimens cannot be transported as hand luggage or checked baggage in passenger aircraft.
Documentation
Documentation required by transporters should be accessible without opening the package. Packages for or from overseas destinations must be accompanied by the necessary documentation, including customs and/or quarantine permits. For Category A substances, a shipper’s declaration must be completed as well as a consignment note or airway bill. Technical names of pathology specimens along with proper shipping names must be included in the shipper’s declaration. For Category B substances, a shipper’s declaration is not essential. However, the consignment note or airway bill is must. Information about dry ice is only required on a shipper’s declaration when the dry ice is used as refrigerant for dangerous goods requiring shipper’s declaration (Category A substances). When the shipper’s declaration is not required, the information about dry ice on the waybill or consignment note should include the name and quantity of goods, proper shipping name, hazard label, UN number (UN 1845), and net weight of dry ice in kilograms for each package.


